Abstract
Venetoclax is a BH3-mimetic small molecule that selectively inhibits the anti-apoptotic protein BCL-2 and triggers cell death. While it is only approved for Acute Myeloid Leukemia (AML) and Chronic Lymphocytic Leukemia (CLL), its use in Multiple Myeloma (MM) in clinical trials has demonstrated excellent activity as a single agent and in combination with other therapies, particularly in patients carrying the t(11;14) translocation involving CCND1 (Kumar et al, Lancet Oncol 2020; Ehsan et al, J Hematol 2021).
In this study, we sought to investigate the genomic determinants of response to venetoclax in a real-world setting outside of clinical trials. In an IRB-approved retrospective review of the Mount Sinai Electronic Medical Record, we identified 58 MM patients who received Venetoclax between 2017 and 2022. Most of these patients received Venetoclax in combination with other therapeutics, including the monoclonal antibody Daratumumab, proteasome inhibitors (PI), immune-modulatory drugs (IMID) and the HDAC inhibitor Panobinostat, and only 5 received Venetoclax as single agent. Patients had received a median of 4 previous lines of therapy (range 1-18).
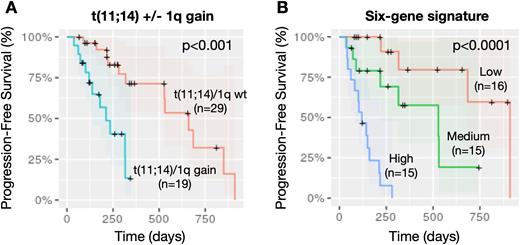
The median progression-free survival (PFS) was 7.13 months, with an ORR of 53% (defined as PR or better). Most patients exhibited the t(11;14) translocation of CCND1 (n=48, 83%), which was likely used as a biomarker for patient selection, given its association with better response to the drug as suggested by previous literature. Median PFS in t(11;14) patients was 7.41 months vs 4 months in patients in the non-t(11;14) group (p=0.07). Further stratification based on gain of 1q revealed that patients with t(11;14) and concurrent gain of 1q (n=19; 39.5%) had significantly worse PFS than patients with t(11;14) alone (HR=5.4; 95% CI: 1.9-15.1; p<0.001) (Fig. 1A). These findings were confirmed also in multivariate analysis including potential confounding variables such as age, sex, race, previous lines of therapy, and ISS.
While t(11;14) was associated with a more durable response, we observed cases of fast progression in the t(11;14) group, as well as cases with durable response in the non-t(11;14) group, indicating that other markers should be investigated for better identification of patients who are more likely to benefit from this treatment.
RNA-seq data was available for 46 of the 58 patients, which we analyzed to determine potential gene expression markers of response. First, we evaluated markers proposed in previous studies, such as BCL2, MCL1 and the BCL2/BCL2L1 and BCL2/MCL1 ratios, stratifying patients based on median PFS. Our analysis showed a trend towards higher expression of BCL2 (p=0.07) and the BCL2/MCL1 ratio (p=0.07), as well as significant up-regulation of the BCL2/BCL2L1 ratio in patients with higher PFS (p<0.05). No significant difference was observed in the expression of MCL1.
To further determine potential novel markers of response, we performed elastic-net regularized regression to predict PFS, which identified a predictive signature of 6 genes (ATP1B3, MYL2, CNR1, FKBPL, LRRK2-DT, LINC02541). The Kaplan-Meier estimator showed significant stratification of patients according to the signature, where up-regulation corresponded to significantly shorter PFS compared with medium expression and down-regulation (p<0.0001) (Fig. 1B). Furthermore, the signature was significantly associated with depth of response, where up-regulation identified non-responders (less than PR; n=22) vs responders (PR and above; n=23) (p<0.05). To validate the predictive power of this signature, we analyzed a dataset with 20 RNA-seq samples from patients who received Venetoclax monotherapy from a recent independent study (Gupta et al., Blood 2021). Since PFS data was not available, we evaluated the signature using Best Response to stratify patients in responders (PR and above; n=10) and non-responders (less than PR; n=10). Concordantly with the results obtained on our dataset, up-regulation of the signature significantly identified non-responders (p=0.01).
In conclusion, here we report that gain of 1q is associated with shorter response to venetoclax in MM, including patients with t(11;14), and the identification of a novel six-gene signature that predicts response to the drug in terms of both duration and depth of response.
Disclosures
McCafferty:Sema4: Current Employment. Newman:Sema4: Current Employment. Kern:Sema4: Current Employment. Corrigan:Sema4: Current Employment. Hantash:Sema4: Current Employment. Rossi:gsk: Consultancy; sanofi: Consultancy; adaptive: Consultancy; BMS: Consultancy; janssen: Consultancy. Rodriguez:Bristol Myers Squibb: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Janssen: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Takeda: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Amgen: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Karyopharm Therapeutics: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau. Chari:Amgen: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Novartis Pharmaceuticals: Research Funding; Celgene: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Takeda: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Seattle Genetics: Membership on an entity's Board of Directors or advisory committees, Research Funding; Array Biopharma: Research Funding; Glaxo Smith Klein: Research Funding; Bristol Myers Squibb: Consultancy; Pharmacyclics: Research Funding; Karyopharm: Consultancy, Membership on an entity's Board of Directors or advisory committees; Sanofi: Membership on an entity's Board of Directors or advisory committees; Oncoceutics: Research Funding; Janssen: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding. Cho:BMS/Celgene: Other: Receive laboratory research support from the above companies. Salary value is less than $10,000 per company., Research Funding; Takeda: Other: Receive laboratory research support from the above companies. Salary value is less than $10,000 per company., Research Funding. Jagannath:Legend Biotech: Consultancy; BMS: Consultancy; Takeda: Consultancy; Karyopharm: Consultancy; Janssen Pharmaceuticals: Consultancy; Sanofi: Consultancy. Richter:Secura Bio: Consultancy, Honoraria; Oncopeptides: Consultancy, Honoraria; Takeda: Consultancy; BMS/Celgene: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau.
OffLabel Disclosure:
Venetoclax is a BCL2 inhibitor not yet approved for multiple myeloma. The abstract discusses markers of response to the drug in myeloma patients treated off-label at Mount Sinai, NY.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal